| The role of magnesium ions in bone regeneration involves the canonical Wnt signaling pathway Publication date: Available online 8 June 2019 Source: Acta Biomaterialia Author(s): Chu-Chih Hung, Amy Chaya, Kai Liu, Konstantinos Verdelis, Charles Sfeir AbstractMagnesium (Mg)-based implants have become of interest to both academia and the medical industry. The attraction largely is due to Mg's biodegradability and ability to enhance bone healing and formation. However, the underlying mechanism of how Mg regulates osteogenesis is still unclear. Based on our previous in vivo and molecular signaling work demonstrating the osteogenic effect of Mg, the current study aims to extend this work at the molecular level especially that we also observed and quantified mineral deposits in the bone marrow space in a rabbit ulna fracture model with Mg plates and screws. Histological analysis and quantitative results of micro-CT showed mineralized deposition and a significant increase in bone volume at 8 weeks and 16 weeks post-operative. These in vivo results led us to focus on studying the effect of Mg2+ on human bone marrow stromal cells (hBMSCs). The data presented in this manuscript demonstrate the activation of the canonical Wnt signaling pathway in hBMSCs when treated with 10 mM Mg2+. With additional Mg2+ present, the protein expression of active β-catenin was significantly increased to a level similar to that of the positive control. Immunocytochemistry and the increased expression of LEF1 and Dkk1, downstream target genes that are controlled directly by active β-catenin, demonstrated the protein translocation and the activation of transcription. Taken together, these data suggest that Mg2+ induces an osteogenic effect in the bone marrow space by activating the canonical Wnt signaling pathway, which in turn causes BMSCs to differentiate toward the osteoblast lineage. Graphical abstract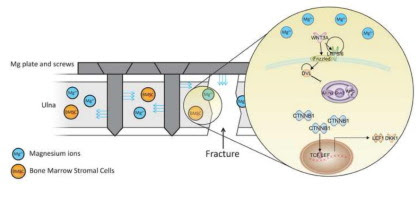 |
|
Publication date: Available online 7 June 2019
Source: Acta Biomaterialia
Author(s): Mohammad Shahin, Khurram Munir, Cuie Wen, Yuncang Li
Abstract
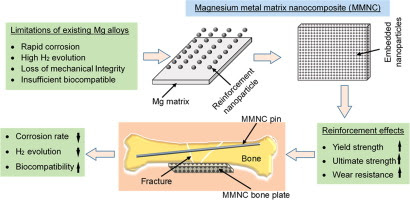
Magnesium (Mg) and some of its alloys have attracted extensive interests for biomedical applications as they exhibit biodegradability and low elastic modulus that is closer to natural bones than the currently used metallic implant materials such as titanium (Ti) and its alloys, stainless steels, and cobalt-chromium (Co-Cr) alloys. However, the rapid degradation of Mg alloys and loss of their mechanical integrity before sufficient bone healing impede their clinical application. Our literature review shows that magnesium matrix nanocomposites (MMNCs) reinforced with nanoparticles possess enhanced strength, high corrosion resistance, and good biocompatibility. This article provides a detailed analysis of the effects of nanoparticle reinforcements on the mechanical properties, corrosion behavior, and biocompatibility of MMNCs as promising biodegradable implant materials. The governing equations to quantitatively predict the mechanical properties and underlying synergistic strengthening mechanisms in MMNCs are elucidated. The potential, recent advances, challenges and future research directions in relation to nanoparticles reinforced MMNCs are highlighted.
Statement of significance
Critically reviewing magnesium metal matrix nanocomposites (MMNCs) for the biomedical application.
Clear definitions of strengthening mechanisms using reinforcement particle in the magnesium matrix, as there were controversial in governing equations of strengthening parameters.
Providing better understanding of the effect of particle size, volume fraction, interfacial bonding, and uniform dispersion of reinforcement particles on MMNCs.
Graphical abstract
|
Publication date: Available online 6 June 2019
Source: Acta Biomaterialia
Author(s): Kiersten E. Scott, Kevin Rychel, Sural Ranamukhaarachchi, Padmini Rangamani, Stephanie I. Fraley
Abstract
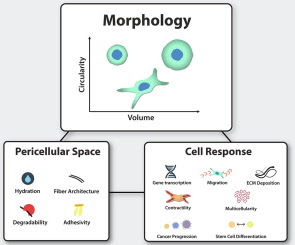
Cells reside in a complex three-dimensional (3D) microenvironment where physical, chemical, and architectural features of the pericellular space regulate important cellular functions like migration, differentiation, and morphogenesis. A major goal of tissue engineering is to identify which properties of the pericellular space orchestrate these emergent cell behaviors and how. In this review, we highlight recent studies at the interface of biomaterials and single cell biophysics that are lending deeper insight towards this goal. Advanced methods have enabled the decoupling of architectural and mechanical features of the microenvironment, revealing multiple mechanisms of adhesion and mechanosensing modulation by biomaterials. Such studies are revealing important roles for pericellular space degradability, hydration, and adhesion competition in cell shape, volume, and differentiation regulation.
Statement of significance
Cell fate and function are closely regulated by the local extracellular microenvironment. Advanced methods at the interface of single cell biophysics and biomaterials have shed new light on regulators of cell-pericellular space interactions by decoupling more features of the complex pericellular milieu than ever before. These findings lend deeper mechanistic insight into how biomaterials can be designed to fine-tune outcomes like differentiation, migration, and collective morphogenesis.
Graphical abstract
|
Publication date: Available online 6 June 2019
Source: Acta Biomaterialia
Author(s): Kevin C. Chan, Yu Yu, Shuk Han Ng, Heather K. Mak, Yolanda W.Y. Yip, Yolandi van der Merwe, Tianmin Ren, Jasmine S.Y. Yung, Sayantan Biswas, Xu Cao, Ying Chau, Christopher K.S. Leung
Abstract
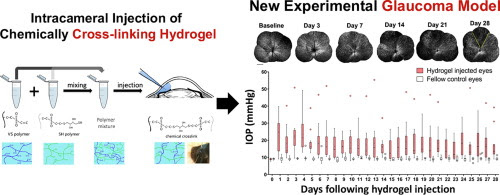
Investigation of neurodegeneration in glaucoma, a leading cause of irreversible blindness worldwide, has been obfuscated by the lack of an efficient model that provides chronic, mild to moderate elevation of intraocular pressure (IOP) with preservation of optical media clarity for long term, in vivo interrogation of the structural and functional integrity of the retinal ganglion cells (RGCs). Here, we designed and formulated an injectable hydrogel based on in situ cross-linking of hyaluronic acid functionalized with vinylsulfone (HA-VS) and thiol groups (HA-SH). Intracameral injection of HA-VS and HA-SH in C57BL/6J mice exhibited mild to moderate elevation of IOP with daily mean IOP ranged between 14±3 and 24±3 mmHg, which led to progressive, regional loss of RGCs evaluated with in vivo, time-lapse, confocal scanning laser ophthalmoscopy; a reduction in fractional anisotropy in the optic nerve and the optic tract projected from the eye with increased IOP in diffusion tensor magnetic resonance imaging; a decrease in positive scotopic threshold response in electroretinography; and a decline in visual acuity measured with an optokinetic virtual reality system. The proportion of RGC loss was positively associated with the age of the animals, and the levels and the duration of IOP elevation. The new glaucoma model recapitulates key characteristics of human glaucoma which is pertinent to the development and pre-clinical testing of neuroprotective and neuroregenerative therapies.
Statement of Significance
A new model to study chronic neurodegeneration in glaucoma has been developed via intracameral injection of a specifically designed hyaluronic acid functionalized with vinylsulfone and thiol groups for cross-linking. Intracameral injection of the chemically cross-linked hydrogel generates mild to moderate IOP elevation, resulting in progressive degeneration of the retinal ganglion cells, optic nerve, and optic tract, and a decline in visual function. The model recapitulates the key features of neurodegeneration in human glaucoma, which will facilitate and expedite the development of neuroprotective and neuroregenerative therapies.
Graphical abstract
|
Publication date: Available online 6 June 2019
Source: Acta Biomaterialia
Author(s): May Griffith
Publication date: Available online 6 June 2019
Source: Acta Biomaterialia
Author(s): Jaywant Phopase
Publication date: Available online 6 June 2019
Source: Acta Biomaterialia
Author(s): Yong Mao, Tyler Hoffman, Sandeep Dhall, Amit Singal, Malathi Sathyamoorthy, Alla Danilkovitch, Joachim Kohn
Abstract
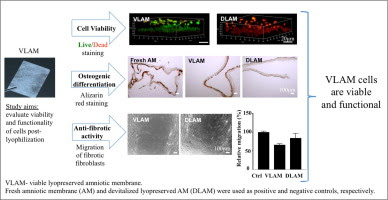
Human amniotic membrane (AM) has intrinsic anti-inflammatory, anti-fibrotic and antimicrobial properties. Tissue preservation methods have helped to overcome the short shelf life of fresh AM allowing "on demand" use of AM grafts. Cryopreserved AM that retains all native tissue components, including viable cells, has clinical benefits in treating chronic wounds. However, cryopreservation requires ultra-low temperature storage, limiting the use of cryopreserved products. To overcome this limitation, a new lyopreservation method has been developed for ambient storage of living tissues. The goal of this study was to investigate the viability and functionality of AM cells following lyopreservation. Fresh AM and devitalized lyopreserved AM (DLAM) served as positive and negative controls, respectively. Using live/dead staining, we confirmed the presence of living cells in viable lyopreserved AM (VLAM) and showed that these cells persisted up to 21 days in culture medium. The functionality of cells in VLAM was assessed by their differentiation potential and anti-fibrotic activity in vitro. With osteogenic induction, cells in VLAM deposited calcium within the membrane, a marker of osteogenic cells, in a time-dependent manner. The migration of human lung fibrotic fibroblasts in a scratch wound assay was reduced significantly in the presence of VLAM-derived conditioned medium. Quantitative PCR analyses indicated that VLAM reduced the expression of pro-fibrotic factors such as type I collagen and increased the expression of anti-fibrotic factors such as hepatocyte growth factor and anti-fibrotic microRNA in fibrotic fibroblasts. Taken together, these results demonstrate that endogenous cells in VLAM remain viable and functional post-lyophilization.
Statement of Significance
This study, for the first time, provides direct evidence showing that tissue viability and functional cells can be preserved by lyophilization. Similar to fresh amniotic membrane (AM), viable lyopreserved AM (VLAM) retains viable cells for extended periods of time. More importantly, these cells are functional and maintain their osteogenic differentiation potential and anti-fibrotic activity. Our results confirmed that the novel lyophilization method preserves tissue viability.
Graphical abstract
|
Publication date: Available online 5 June 2019
Source: Acta Biomaterialia
Author(s): Alexander P. Morrell, Hayley Floyd, J. Frederick W. Mosselmans, Liam M. Grover, Hiram Castillo-Michel, Edward Davis, Julia E. Parker, Richard A. Martin, Owen Addison
Abstract
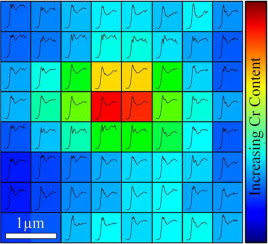
Biological exposures to micro- and nano-scale exogenous metal particles generated as a consequence of in-service degradation of orthopaedic prosthetics can result in severe adverse tissues reactions. However, individual reactions are highly variable and are not easily predicted, due to in part a lack of understanding of the speciation of the metal-stimuli which dictates cellular interactions and toxicity. Investigating the chemistry of implant derived metallic particles in biological tissue samples is complicated by small feature sizes, low concentrations and often a heterogeneous speciation and distribution. These challenges were addressed by developing a multi-scale two-dimensional X-ray absorption spectroscopic (XAS) mapping approach to discriminate sub-micron changes in particulate chemistry within ex-vivo tissues associated with failed CoCrMo total hip replacements (THRs). As a result, in the context of THRs, we demonstrate much greater variation in Cr chemistry within tissues compared with previous reports. Cr compounds including phosphate, hydroxide, oxide, metal and organic complexes were observed and correlated with Co and Mo distributions. This variability may help explain the lack of agreement between biological responses observed in experimental exposure models and clinical outcomes. The multi-scale 2D XAS mapping approach presents an essential tool in discriminating the chemistry in dilute biological systems where speciation heterogeneity is expected.
Significance
Metal implants are routinely used in healthcare but may fail following degradation in the body. Although specific implants can be identified as 'high-risk', our analysis of failures is limited by a lack of understanding of the chemistry of implant metals within the peri-prosthetic milieu. A new approach to identify the speciation and variability in speciation at sub-micron resolution, of dilute exogenous metals within biological tissues is reported; applied to understanding the failure of metallic (CoCrMo) total-hip-replacements widely used in orthopedic surgery. Much greater variation in Cr chemistry was observed compared with previous reports and included phosphate, hydroxide, oxide, metal and organic complexes. This variability may explain lack of agreement between biological responses observed in experimental exposure models and clinical outcomes.
Graphical abstract
|
Publication date: Available online 5 June 2019
Source: Acta Biomaterialia
Author(s): Scott C. Grindy, Dmitry Gil, Jeremy V. Suhardi, Orhun K. Muratoglu, Hany Bedair, Ebru Oral
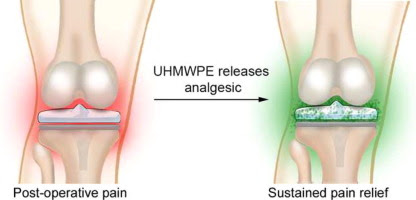
Abstract
Total joint replacement is a widely used and successful surgical approach. Approximately 7 million US adults are currently living with a hip or knee replacement. However, the surgical procedures for total joint replacement are associated with significant postoperative pain, and current strategies do not adequately address this pain, which leads to patient dissatisfaction, reduced mobility, and increased risk of opioid addiction. We hypothesized that the ultra-high-molecular-weight polyethylene (UHMWPE)-bearing surfaces used in total joint prosthetics could provide sustained release of the local anesthetic bupivacaine, to provide relief from joint pain for an extended period of time after surgery. In this paper, we describe the production of bupivacaine-loaded UHMWPE (BPE) and measure the in vitro bupivacaine release kinetics of BPE. We found that bupivacaine could be released from BPE at clinically relevant rates for up to several days and that BPE possesses antibacterial effects. Therefore, bupivacaine-loaded UHMWPE is a promising material for joint replacement prostheses, and future studies will evaluate its safety and efficacy in in vivo models.
Statement of Significance
Total joint replacement is associated with significant pain and risk of infection. In our paper, we introduce bupivacaine-loaded ultra-high molecular weight polyethylene (BPE) which releases bupivacaine, a pain-treating drug, at levels comparable to currently-used doses. Additionally, BPE inhibits growth of infection-causing bacteria. Therefore, BPE may be able to reduce both post-surgical pain and risk of infection, treating two of the most prominent complications associated with total joint replacement. To our knowledge, this is the first development of a material that can address both complications, and devices incorporating BPE would represent a significant advance in joint arthroplasty prosthetics. More generally, the incorporation of therapeutic agents into ultra-high molecular weight polyethylene could impact many orthopedic procedures due its ubiquity.
Graphical abstract
|
Publication date: June 2019
Source: Acta Biomaterialia, Volume 91
Author(s): Andres Ruland, Kerry J. Gilmore, Luciana Y. Daikuara, Cormac D. Fay, Zhilian Yue, Gordon G. Wallace
Abstract
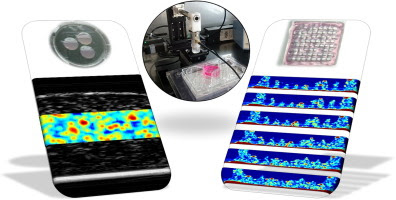
In the present work we have revisited the application of quantitative ultrasound imaging (QUI) to cellular hydrogels, by using the reference phantom method (RPM) in combination with a local attenuation compensation algorithm. The investigated biological samples consisted of cell-laden collagen hydrogels with PC12 neural cells. These cell-laden hydrogels were used to calibrate the integrated backscattering coefficient (IBC) as a function of cell density, which was then used to generate parametric images of local cell density. The image resolution used for QUI and its impact on the relative IBC error was also investigated. Another important contribution of our work was the monitoring of PC12 cell proliferation. The cell number estimates obtained via the calibrated IBC compared well with data obtained using a conventional quantitative method, the MTS assay. Evaluation of spectral changes as a function of culture time also provided additional information on the cell cluster size, which was found to be in close agreement with that observed by microscopy. Last but not least, we also applied QUI on a 3D printed cellular construct in order to illustrate its capabilities for the evaluation of bioprinted structures.
Statement of Significance
While there is intensive research in the areas of polymer science, biology, and 3D bio-printing, there exists a gap in available characterisation tools for the non-destructive inspection of biological constructs in the three-dimensional domain, on the macroscopic scale, and with fast data acquisition times. Quantitative ultrasound imaging is a suitable characterization technique for providing essential information on the development of tissue engineered constructs. These results provide a detailed and comprehensive guide on the capabilities and limitations of the technique.
Graphical abstract
|
Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου